Abstract
Introduction: CPX-351, a liposomal formulation of daunorubicin and cytarabine is FDA-approved for elderly patients with newly diagnosed acute myeloid leukemia (AML) with myelodysplasia related changes and therapy-related AML based on superior response and survival compared to conventional cytarabine plus daunorubicin (7+3) (JCO, 2018). Treatment for blast-phase myeloproliferative neoplasm (MPN-BP) is limited to 7+ 3 or venetoclax plus hypomethylating agents (HMA) with complete remission (CR) and CR with incomplete count recovery (CRi) rate of 59% and 44%, respectively (Leukemia 2018, AJH 2021). CPX-351 remains a therapeutic consideration, however its efficacy in MPN-BP is unknown. Therefore, our primary objective was to evaluate the efficacy of CPX-351 in patients with newly diagnosed MPN-BP.
Methods: The current study comprises of 10 consecutive patients with MPN-BP treated with CPX-351 outside the context of a clinical trial at the Mayo Clinic between 2018 and 2021. Diagnosis of MPN-BP required the presence of ≥20% blasts in either the peripheral blood or bone marrow. All patients received CPX-351, daunorubicin 44mg/cytarabine 100mg/m² I/V on days 1, 3 and 5. Bone marrow biopsy was obtained after cycle 1 with response assessed according to the 2017 European Leukemia Net (ELN) criteria. Cytogenetic and molecular studies were performed by conventional karyotype, and next-generation sequencing of a 42-gene panel, respectively.
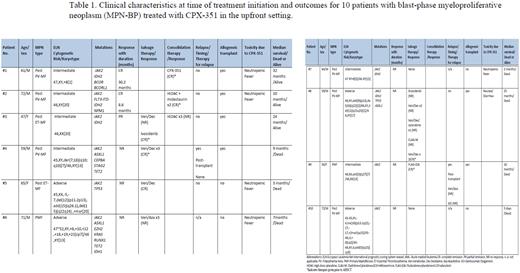
ResultsPatient characteristics A total of 10 patients with MPN-BP (median age 62 years, range 45-73; 70% males) received CPX-351 in the upfront setting. Antecedent MPN included post-polycythemia vera myelofibrosis (post-PV MF) (50%), primary myelofibrosis (30%), and post-essential thrombocythemia myelofibrosis (post-ET MF) (20%). All patients were JAK2 mutated; other mutations included IDH1/2 (67%), ASXL1 (33%), TP53 (22%), and TET2 (22%). Cytogenetics were abnormal in 7 of 9 (78%) evaluable patients, with complex karyotype in 44%. Patient characteristics at time of leukemic transformation, treatment details, and response rates are shown in Table 1.
Response and toxicity Treatment-emergent toxicity included neutropenic fever in half of patients (n=5), resulting in mortality in one patient. Two of ten (20%) patients achieved CR following CPX-351 induction therapy. Patient 1 was a 61-year-old male with antecedent post-PV MF and trisomy 8, IDH2 and BCOR mutated, achieved CR following two cycles of CPX-351 induction therapy. He was bridged to allogeneic hematopoietic stem cell transplant (AHSCT) following one cycle of CPX-351 consolidation and remains relapse-free thirty-two months after leukemic transformation. The second patient was a 72-year-old male with antecedent post-PV MF, FLT3-ITD, NPM1 and IDH2 mutated with normal karyotype, received one cycle of CPX-351 plus midostaurin and achieved CR. Subsequently, he received two cycles of high dose cytarabine plus midostaurin consolidation followed by AHSCT and remains disease-free ten months following diagnosis.
Second-line therapy following failure of CPX-351 was pursued in 6 patients and included venetoclax plus HMA (n=4), FLAG-IDA (n=1), and enasidenib (n=1). Two of four patients treated with venetoclax plus HMA achieved CR, one of whom was bridged to AHSCT, and an additional patient achieved CR and underwent AHSCT following salvage therapy with FLAG-IDA. Notably, two patients attained CR with ivosidenib (Patient #3) and decitabine plus venetoclax (Patient #8), respectively, after failure of second line and subsequent therapies, and also proceeded to AHSCT
Survival At a median follow-up of 9.5 months (range; 0.2-32 months), seven patients have died from disease progression (n=5) and sepsis (n=2). Median overall survival was 12.5 months (95% CI, 3-25 months) and was longer in six patients that were transplanted (25 months vs 2.5 months without AHSCT, p=0.001).
Conclusion: The current study demonstrates limited therapeutic activity of CPX-351 in newly diagnosed MPN-BP with CR rate of 20% in comparison to 47.7%, in secondary (post-MDS and therapy-related) AML (NCT01696084) (JCO, 2018). Furthermore, the value of AHSCT for long-term survival in patients with MPN-BP is underscored.
Disclosures
Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding. Mangaonkar:Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.